Description
Global Deep Venous Disease Treatment Devices Market: Industry Overview
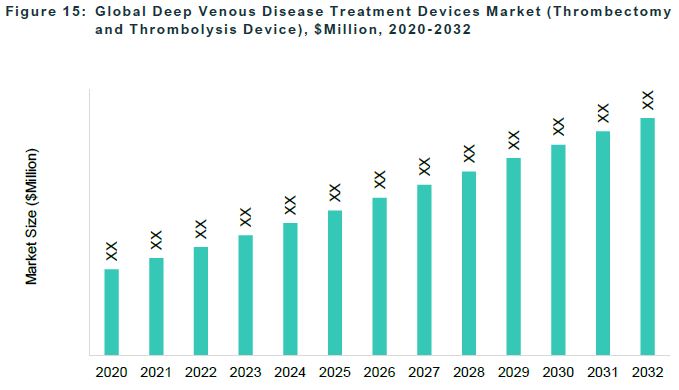
The global deep venous disease treatment devices market was valued at $1,119.3 million in 2022 and is anticipated to reach $2,419.2 million by 2032, witnessing a CAGR of 7.72% during the forecast period 2023-2032.
The market is driven by factors such as the upsurge in the incidence of deep vein diseases leading to increased demand for treatment devices, increasing awareness of preventative devices leading to the growth of deep venous disease devices in the market, and a rise in diagnosis of deep venous diseases in hospitals leading to increased use of treatment devices.
Market Lifecycle Stage
The global deep venous disease treatment devices market is in the developed phase. The technological advancements in vascular stents and the development of advanced intermittent pneumatic compression (IPC) devices for prophylaxis of diseases are some of the major opportunities in the global deep venous disease treatment devices market.
Impact of COVID-19
The COVID-19 pandemic has impacted the global healthcare ecosystem in different manners. Among the sectors ofthe healthcare ecosystem that got less impacted by the pandemic are the deep venous disease treatment devices.
Since the number of patients hospitalized during the pandemic was at its peak, the risk of patients developing different deep venous diseases got highly increased and resulted in more cases of diseases such as deep vein thrombosis. Hence, the COVID-19 pandemic has resulted in an increase in treatment and prevention devices for deep venous diseases.
Market Segmentation:
Segmentation 1: by Product Type
• Thrombectomy and Thrombolysis Device
• Inferior Vena Cava (IVC) Filter
• Peripheral Vascular Stent
• PTA Balloon Catheter
• Accessory Device
• Compression Device/Stockings
The accessory device is the largest segment among the products, followed by the thrombectomy and thrombolysis device segment.
Segmentation 2: by End User
• Hospitals and Clinics
• Ambulatory Surgical Centres
• Home-Care
The global deep venous disease treatment devices market (by end user) is expected to be dominated by the hospitals and clinics segment.
Segmentation 3: by Region
• North America – U.S., Canada
• Europe – Germany, U.K., France, Italy, Spain, and Rest-of-Europe
• Asia-Pacific – China, Japan, India, Australia, South Korea, and Rest-of-Asia-Pacific
• Rest-of-the-World – Brazil, South Africa, and Rest-of-Rest-of-the-World
The global deep venous disease treatment devices market (by region) is dominated by the North America segment.
Recent Developments in the Global Deep Venous Disease Treatment Devices Market
• In January 2023, Innova Vascular, Inc. submitted the 510(k) Premarket Notification for its thrombectomy devices for the treatment of peripheral vascular disease to the U.S. Food and Drug Administration (FDA).
• In January 2023, Penumbra, Inc. launched its “Lightning Flash,” a mechanical thrombectomy system with U.S. FDA clearance.
• In December 2022, Zylox-Tonbridge Medical Technology received approval from China’s National Medical Products Administration (NMPA) for the commercialization of its Zylox Octoplus retrievable inferior vena cava (IVC) filter. The company’s product provides a reliable treatment solution for patients with high-risk deep vein thrombosis (DVT).
• In September 2022, Penumbra, Inc. collaborated with Asahi Intecc Co., Ltd. Through this collaboration, the companies aimed to introduce Penumbra, Inc.’s “Indigo Aspiration System” commercially in the Japan market.
• In February 2022, Innova Vascular, Inc. partnered with Cardiovascular Systems, Inc. with the aim of developing a complete line of thrombectomy devices.
• In September 2022, Merit Medical Systems, Inc. commercially launched its “Prelude Roadster Guide Sheath” in the U.S.
• In February 2022, Boston Scientific Corporation announced the acquisition of Baylis Medical Company Inc. Through this acquisition, the company enhanced its product portfolio by adding several products made by Baylis Medical Company Inc., such as transseptal access solutions, guidewires, sheaths, and dilators.
• In January 2022, Akura Medical, Inc., a subsidiary of Shifamed LLC, raised a funding of $25 million in its Series A1 financing round. Through this funding, the company aims to support the development of its next-generation thrombectomy device.
• In July 2021, Surmodics, Inc. announced the acquisition of Vetex Medical Ltd. Through this acquisition, the company aimed to boost its thrombectomy product portfolio.
Demand – Drivers and Limitations
The following are the drivers for the global deep venous disease treatment devices market:
• Upsurge in Incidence of Deep Vein Diseases Leading to Increased Demand for Treatment Devices
• Increasing Awareness of Preventative Devices Leading to the Growth of Deep Venous Disease Devices in the Market
• Rise in Diagnosis of Deep Venous Diseases in Hospitals Leading to Increased Use of Treatment Devices
The market is expected to face some limitations as well due to the following challenges:
• Rise in Drug-Based Treatment for Deep Venous Diseases Hindering the Adoption of Surgical Devices
• Lack of Skilled Professionals Performing Vascular Surgery Expected to Hinder the Market of Surgical Treatment Devices
How can this report add value to an organization?
Offerings: The offerings segment helps the reader understand the different types of deep venous disease treatment devices available in the market. Moreover, the study provides the reader with a detailed understanding of products that fall under the six main segments, i.e., thrombectomy and thrombolysis device, inferior vena cava filter, peripheral vascular stent, PTA balloon catheter, accessory device, and compression device/stockings.
Growth/Marketing Strategy: The global deep venous disease treatment devices market has witnessed major developments by key players operating in the market, such as product launches, business expansions, partnerships, collaborations, mergers and acquisitions, funding activities, and regulatory and legal approvals.
The favored strategy for the companies has been regulatory and legal activities to strengthen their position in the market. For instance, in January 2023, Innova Vascular, Inc. submitted the 510(k) Premarket Notification for its thrombectomy devices for the treatment of peripheral vascular disease to the U.S. Food and Drug Administration (FDA).
Competitive Strategy: The key players in the global deep venous disease treatment devices market analyzed and profiled in the study involve established and emerging players that offer different products for deep venous diseases.
Moreover, a detailed competitive benchmarking of the players operating in the global deep venous disease treatment devices market has been done to help the reader understand the ways in which players stack against each other, presenting a clear market landscape.
Moreover, comprehensive competitive strategies such as partnerships, agreements, collaborations, and mergers and acquisitions will help the reader understand the untapped revenue pockets in the market.
Key Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and analyzing company coverage, product portfolio, and market penetration.
Key Companies Profiled
• Abbott Laboratories
• AndraTec GmbH
• AngioDynamics, Inc.
• Boston Scientific Corporation
• Cardinal Health
• Cook Group
• Innova Vascular, Inc.
• Koninklijke Philips N.V.
• Medtronic plc
• Nipro Corporation
• Penumbra, Inc.
• Stryker Corporation
• Surmodics, Inc. (Vetex Medical Ltd.)
• Teleflex Incorporated
• Terumo Corporation



Reviews
There are no reviews yet.